Banner

Boyle's Law
-
Boyle's Law Definition in Chemistry
 Boyle's law states that the pressure of an ideal gas increases as its container volume decreases.
Boyle's law states that the pressure of an ideal gas increases as its container volume decreases. -
The Simple Gas Laws- Boyle’s Law, Charles’s Law and Avogadro’s Law
 Early scientists explored the relationships among the pressure of a gas (P) and its temperature (T), volume (V), and amount (n) by holding two of the four variables constant (amount and temperature, for example), varying a third (such as pressure), and measuring the effect of the change on the fourth (in this case, volume).
Early scientists explored the relationships among the pressure of a gas (P) and its temperature (T), volume (V), and amount (n) by holding two of the four variables constant (amount and temperature, for example), varying a third (such as pressure), and measuring the effect of the change on the fourth (in this case, volume).
Boyle's Law and snorkelling
-
How Does Boyle's Law Apply to Scuba Diving?
 Boyle's Law explains how the volume of a gas varies with the surrounding pressure. Many aspects of scuba diving physics and dive theory become clear once you understand this simple gas law.
Boyle's Law explains how the volume of a gas varies with the surrounding pressure. Many aspects of scuba diving physics and dive theory become clear once you understand this simple gas law.
-
Diving gas laws
 The Gas Laws are particularly relevant to diving. Having a good understanding of the implications can help make you a safer diver.
The Gas Laws are particularly relevant to diving. Having a good understanding of the implications can help make you a safer diver.
Images
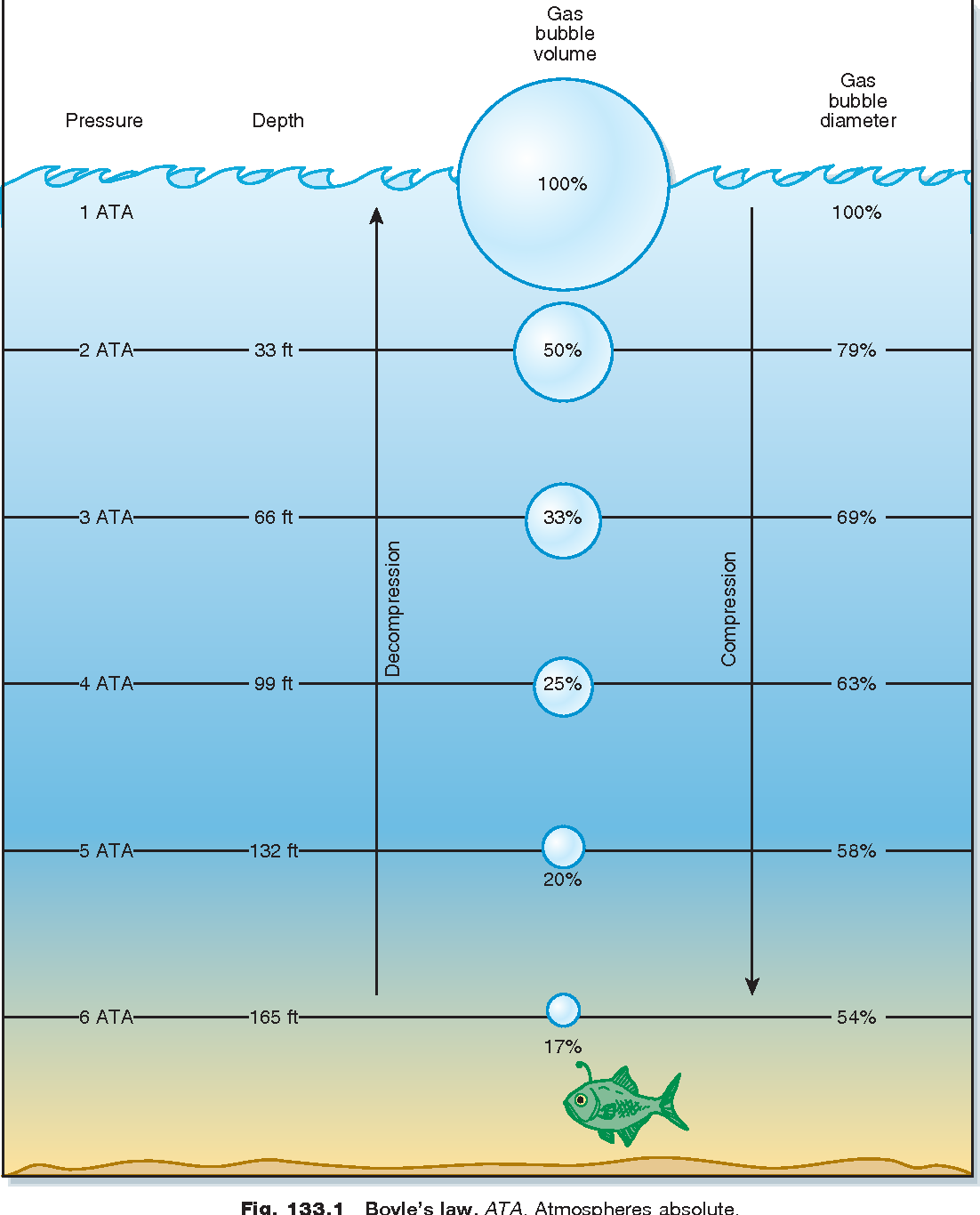
Murphy-Lavoie, H., & Legros, T. (2013). 133 - Dysbarisms, Dive Injuries, and Decompression Illness. https://www.semanticscholar.org/paper/133-Dysbarisms%2C-Dive-Injuries%2C-and-Decompression-Murphy-Lavoie-Legros/5d99599a8155b4ee73d325d69a4dd04931d2b8d3
Boyle's Law pressure on ears

Dive Science: How Boyle’s Law Applies to SCUBA Diving. (2013). Aqua Views | Online Scuba Magazine. https://www.leisurepro.com/blog/scuba-guides/dive-science-boyles-law-applies-scuba-diving/amp/